Beta-Lactams as Selective Chemical Probes for the in Vivo Labeling of Bacterial Enzymes Involved in Cell Wall Biosynthesis, Antibiotic Resistance, and Virulence
10-Sep-2008
Journal of the American Chemical Society, 2008, DOI: 10.1021/ja803349j, published on 10.09.2008
JACS, online article
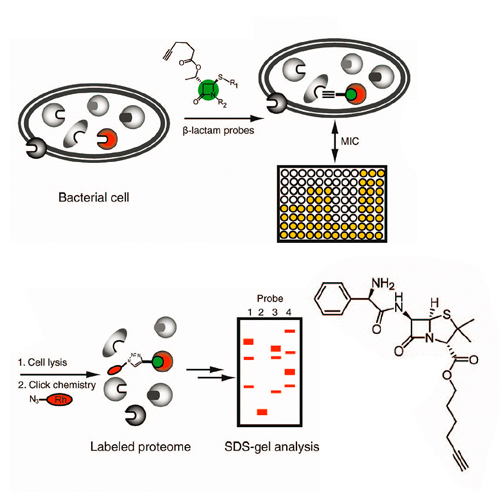
With the development of antibiotic-resistant bacterial strains, infectious diseases have become again a life-threatening problem. One of the reasons for this dilemma is the limited number and breadth of current therapeutic targets for which several resistance strategies have evolved over time. To expand the number of addressable enzyme targets and to understand their function, activity, and regulation, we utilized a chemical proteomic strategy, called activity-based protein profiling (ABPP) pioneered by Cravatt, for the identification of β-lactam-binding enzymes under in vivo conditions. In this two-tiered strategy, we first prepared a selection of conventional antibiotics for labeling diverse penicillin binding proteins (PBPs) and second introduced a new synthetic generation of β-lactam probes, which labeled and inhibited a selection of additional PBP unrelated bacterial targets. Among these, the virulence-associated enzyme ClpP and a resistance-associated β-lactamase were labeled and inhibited by selected probes, indicating that the specificity of β-lactams can be adjusted to versatile enzyme families with important cellular functions.