beta-Lactones as Privileged Structures for the Active-Site Labeling of Versatile Bacterial Enzyme Classes
09-Apr-2008
Angewandte Chemie, 2008, 47, DOI: 10.1002/ anie.200705768 published on 09.04.2008
Angewandte Chemie International Edition, online article
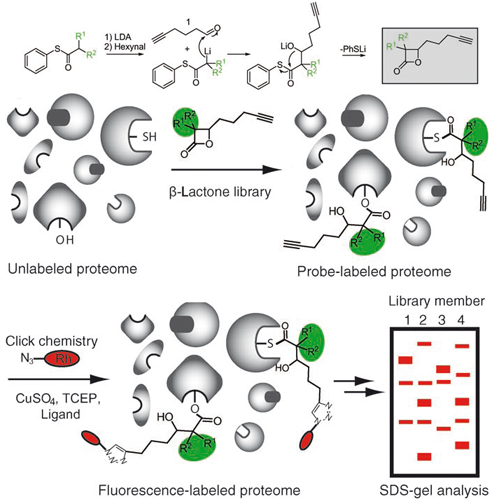
Evolution of multiresistant bacterial strains has meant that infectious diseases once again pose a major threat to public health. Since many antibiotics still target only a limited set of cellular functions, it is a desirable goal to expand the number and breadth of therapeutic targets as well as to gain a deeper understanding of the molecular mechanisms responsible for pathogenesis.To approach this goal, a chemical proteomic strategy (activity-based protein profiling, ABPP), developed by Evans and Cravatt, that uses active-site-directed probes was directly applied to bacterial proteomes. ABPP probes consist of at least two general elements: 1) a reactive group for binding and covalently modifying the active site of a certain enzyme class, and 2) a reporter tag for the detection, enrichment, and identification of probe-labeled proteins.