Total Syntheses and Biological Evaluation of 3-O-Methylfunicone and Its Derivatives Prepared by TMPZnCl·LiCl-Mediated Halogenation and Carbonylative Stille Cross-Coupling
20-Jun-2013
Eur. J. Org. Chem., 2013, 1, 77-83 published on 19.11.2012
Eur. J. Org. Chem.
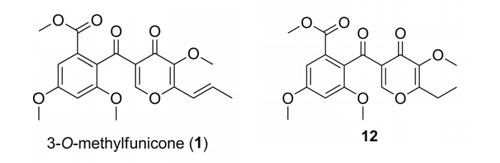
The total syntheses of the natural product 3-O-methylfunicone (1), a member of the funicone class of compounds, and its derivatives is reported. The key reactions in the construction of the biaryl ketone core are a regioselective TMPZnCl•LiCl halogenation and a carbonylative Stille cross-coupling reaction. In addition, the inhibitory activities of the funicones against Y-family DNA polymerase κ (pol κ) and polymerase η (pol η) were determined. We found that 1 and 12 exhibit inhibitory activity against pol η and 1 also against pol κ.