Ultrafast dynamics and temperature effects on the quantum efficiency of the ring-opening reaction of a photochromic indolylfulgide
21-Mar-2008
Journal of Molecular Liquids, 2008, 141, 137-9 published on 21.03.2008
Journal of Molecular Liquids, online article
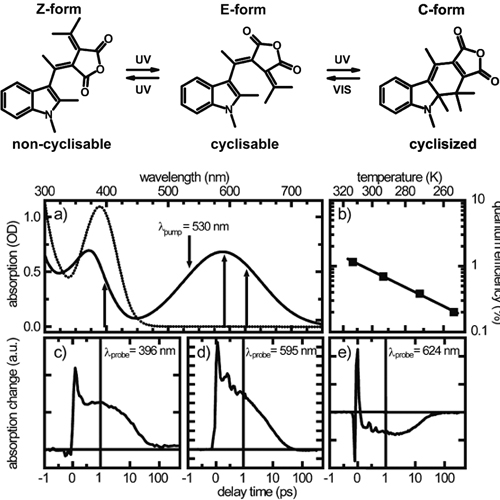
The ultrafast ring-opening reaction of the molecular switch 1,2-Dimethyl-3-indolylfulgide dissolved in acetonitrile is investigated by temperature dependent quantum efficiency measurements and time-resolved transient absorption spectroscopy in the ultraviolet and visible spectral range. The photoreaction is found to be thermally activated with an activation energy of about 1640 cm−1. The transient absorption signal is biexponential with the time constants τ1=0.7 ps and τ2=12 ps. The fast time constant is due to solvation dynamics, while the main component τ2 is attributed to the excited state lifetime and product formation. A long-lived intermediate state in the photoreaction can be excluded.