DNA photodamage: Study of cyclobutane pyrimidine dimer formation in a locked thymine dinucleotide
02-Jul-2010
Spectroscopy, 2010, 24, 309-316 published on 02.07.2010
Spectroscopy
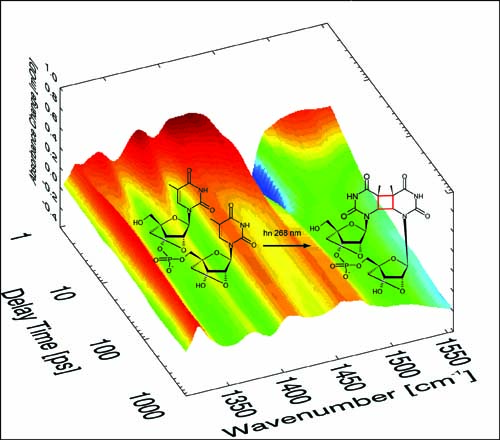
The cyclobutane pyrimidine dimer (CPD) formed between two adjacent thymine bases is the most abundant DNA photolesion induced by UV radiation. The quantum yield of this reaction is on the order of ~1% in DNA. This small quantum yield hampers the study of damage formation in naturally occurring DNA. Investigations with increased accuracy become possible for a locked nucleotide model compound TLpTL which exhibits a quantum yield of about 10% for CPD formation. Time resolved IR spectroscopy on TLpTL and two other DNA model compounds (TpT and (dT)18) reveals that: (i) The absorption changes after ~1 ps are due to CPD photodamage. (ii) The quantum efficiency of CPD formation on the few picosecond time scale equals the quantum efficiency reported in stationary experiments. CPD photodamage formation in the investigated DNA constructs is thus predominantly formed from the primarily photoexcited singlet ππ* state, whereas the triplet channel does not play an essential role.