Light-Triggered Aggregation and Disassembly of Amyloid-Like Structures
27-Dec-2010
Chem. Phys. Chem., 2011, 12, 559 – 562 published on 27.12.2010
Chem. Phys. Chem.
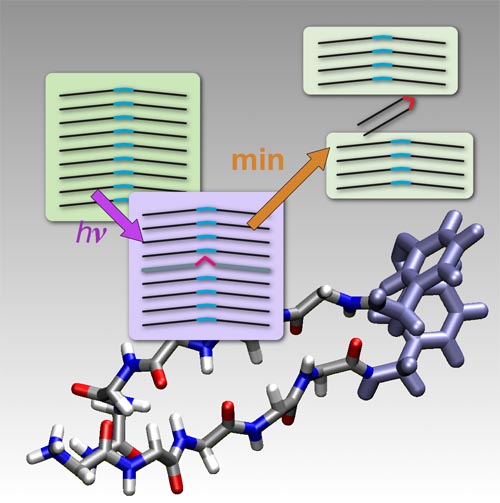
Self-assembly and aggregation of proteins or peptides into amyloid fibrils have attracted wide attention due to their high relevance for a variety of amyloid-related diseases.[1–3] Furthermore, amyloids show interesting material properties which make them ideal candidates for the production of nanostructures and molecular nanobiomaterials, where building blocks may be varied by protein engineering techniques.[4, 5] A rapidly emerging field is the control of aggregation by external means such as pH value or illumination of caged compounds, which allows the investigation of the basic principles of amyloid aggregation and the development of new nanobiomaterials.[6–13] In this context we report on a new peptide system where a chromophore is integrated in the backbone and where illumination not only controls aggregation but the disassembly of amyloid-like structures.