Research Area B
Publications 2013
28-Jan-2014
Complex multi-stage relaxation and reaction pathways after optical excitation of molecules makes the disentanglement of the underlying mechanisms challenging. We present four examples that a new transient spectrometer with excitation fully tunable from the deep UV to the IR and 225 to 1700 nm probing allows for an analysis with greatly reduced ambiguity. The temporal ... READ MORE
28-Jan-2014
We show that two-dimensional Fourier transform spectroscopy can be carried out in a shaper assisted collinear setup comprising fully tunable UV pulse pairs and supercontinuum probe spanning 250 - 720 nm. The potential of the setup is demonstrated on two representative molecules: pyrene and 2,2-diphenyl-5,6-benzo(2H)chromene. Well resolved cross peaks are observed and ... READ MORE
28-Jan-2014
We present a three-color mid-IR setup for vibrational pumprepump-probe experiments with a temporal resolution well below 100 fs and a freely selectable spectral resolution of 20 to 360 cm⁻¹ for the pump and repump. The performance of the spectrometer is demonstrated with transient IR spectra and decay curves of HDO molecules in lithium nitrate trihydrate and ice ... READ MORE
28-Jan-2014
Benzhydryl radicals and cations are reactive intermediates central to the understanding of organic reactivity. They can be generated from benzhydryl halides by UV irradiation. We performed transient absorption measurements over the range from femtoseconds to microseconds to unravel the complete reaction scheme. ... READ MORE
28-Jan-2014
The bandwidth of ultrafast pulses in the UV is limited by the finite acceptance bandwidth of the nonlinear crystals used for their generation. We now show that self-phase modulation of UV pulses in bulk materials leads to large spectral broadening and allows for a significant reduction of the pulse duration. ... READ MORE
16-Dec-2013
The singlet, open-shell singlet and triplet potential energy surfaces (PES) for the peroxo state of a catalytic functional tyrosinase model have been investigated by density functional theory calculations. The broken-symmetry solution exhibits considerable stabilisation over the whole PES but the importance of the triplet state is unravelled as well. ... READ MORE
16-Dec-2013
Kinetics of the reactions of benzhydrylium ions (Aryl2CH+) with the vinylsilanes H2C═C(CH3)(SiR3), H2C═C(Ph)(SiR3), and (E)-PhCH═CHSiMe3 have been measured photometrically in dichloromethane solution at 20 °C. All reactions follow second-order kinetics, and the second-order rate constants correlate linearly with the electrophilicity parameters E of the ... READ MORE
26-Nov-2013
Reactions of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) with silyl enol ethers, silyl ketene acetals, allylsilanes, enamino esters, and diazomethanes have been studied in CH₃CN and CH₂Cl₂solutions. The second-order rate constants for C attack at DDQ (log k C) correlate linearly with the nucleophile-specific parameters N and sɴ and are 2–5 orders of ... READ MORE
26-Nov-2013
Oxygen versus carbon: Azolium enolates were generated by the reactions of N-heterocyclic carbenes (NHCs) with methyl phenyl ketene and characterized by X-ray crystallography. Kinetic studies show that the enolate oxygen is 20 times more nucleophilic than the carbon atom, but under thermodynamic control exclusive C-addition products were formed. ... READ MORE
26-Nov-2013
Kinetics of the reactions of pyridinium, isoquinolinium, and quinolinium ylides with diarylcarbenium ions, quinone methides, and arylidene malonates (reference electrophiles) have been studied in dimethylsulfoxide solution by UV–vis spectroscopy. The second-order rate constants (log k₂) were found to correlate linearly with the electrophilicities E of the ... READ MORE
26-Nov-2013
The 1H NMR chemical shifts of the C(α)— H protons of arylmethyl triphenylphosphonium ions in CD2Cl2 solution strongly depend on the counteranions X−. The values for the benzhydryl derivatives Ph2CH—PPh3+ X−, for example, range from δH=8.25 (X−=Cl−) over 6.23 (X−=BF4−) to 5.72 ppm (X−=BPh4−). Similar, albeit weaker, ... READ MORE
26-Nov-2013
Intriguing imidazolidinones! Inspired by noncovalent interactions in proteins, a series of electronically distinct MacMillan catalysts were designed. The effect of electronic modulation on ground state conformation, reactivity, and performance was studied in a catalytic setting with intriguing outcomes. ... READ MORE
26-Nov-2013
What can attack at carbon? With a nucleophilicity N≈7 for attack at the azomethine carbon, formaldehyde hydrazones can undergo noncatalyzed reactions at room temperature with electrophiles that have a reactivity parameter E that is greater than −12 ... READ MORE
26-Nov-2013
Pyridinium salts Py+-CH2-EWG (EWG = CO2Et, CONEt2, CN, COMe, COPh) reacted with Michael acceptors Ar-CH=C(CO2Et)(Acc) (Acc = CO2Et, COMe, SO2Me, CONH2) at ambient temperature in the presence of base to give [3 + 2]-cycloadducts by stepwise [3 + 2]-cycloaddition of the ... READ MORE
20-Jun-2013
The generation of carbocations by laser flash photolysis of suitable precursors provides information about their reactivities toward nucleophiles on the nanosecond and microsecond time scale that cannot be obtained by conventional methods. We discuss the requirements that must be met by the precursors to achieve sufficient yields of the carbocations under the ... READ MORE
19-Jun-2013
The nucleophilicity parameters N and sɴ, as defined by the linear free-energy equation log k(20 °C) = sɴ (N + E), of the 2-imidazolines and the related N-heterocyclic compounds have been determined by studying the rates of their reactions with differently substituted benzhydrylium ions in dichloromethane at 20 °C by stopped-flow or laser flash photolysis ... READ MORE
19-Jun-2013
. First-order rate constants k1 for the trapping of various donor- and acceptor-substituted benzhydrylium ions in mixtures of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and water ranging from 50 to 99% HFIP (w/w) were determined by laser flash photolytic generation of benzhydrylium ions from benzhydryl triarylphosphonium salts in ... READ MORE
19-Jun-2013
Rate constants for the reactions of benzaldehyde-derived iminium ions with C-nucleophiles (enamines, silylated ketene acetals, and enol ethers) have been determined photometrically in CH3CN solution and used to determine the electrophilicity parameters E of the cations defined by the correlation log k20°C = ... READ MORE
19-Jun-2013
Rates of hydride transfer from several hydride donors to benzhydrylium ions have been measured at 20 °C and used for the determination of empirical nucleophilicity parameters N and sN according to the linear free energy relationship log k20 °C=sN(N+E). Comparison of the ... READ MORE
19-Jun-2013
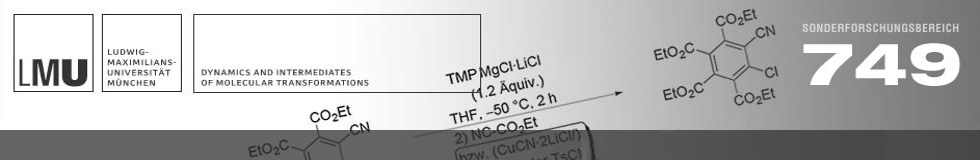
The functionalization of diazines is of great importance, because these N-heterocycles are present in numerous natural products, pharmaceuticals, and agrochemicals. They also find applications in materials science and polymer chemistry. The directed metalation and further functionalization of these electron-deficient N-heterocycles can be realized with ate bases and ... READ MORE
19-Jun-2013
Kinetics and mechanisms of transition-metal free reactions of furyl, thienyl and indolyl trifluoroborates with benzhydrylium (Ar2CH+) and iminium (Me2N+═CHR) ions have been investigated. In contrast to common belief, substitutions at CH positions are often faster than ipso-substitutions of the BF3K group, ... READ MORE
19-Jun-2013
The kinetics of the reactions of carbanions, carboxylate anions, and primary and secondary amines with acylating agents were studied in acetonitrile and dimethyl sulfoxide (DMSO) under first-order conditions by UV/Vis spectrophotometry and conductimetry. Whereas reactions of a large variety of nucleophiles with ordinary carbanions and Michael acceptors generally ... READ MORE
19-Jun-2013
Bond cleavage and bond formation are central to organic chemistry. Carbocations play a key role in our understanding of nucleophilic substitution reactions that involve both processes. The precise understanding of the mechanism and dynamics of the photogeneration of carbocations and carbon radicals is therefore an important quest. In particular, the role of electron ... READ MORE