Research Area B
Publications 2012
28-Nov-2012
The key steps in most organocatalytic cyclizations are the reactions of electrophiles with nucleophiles. Their rates can be calculated by the linear free-energy relationship log k(20 °C) = sN(E + N), where electrophiles are characterized by one parameter (E) and nucleophiles are characterized by the solvent-dependent nucleophilicity (N) and sensitivity (sN) ... READ MORE
28-Nov-2012
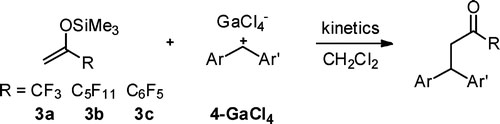
The fluorinated trimethylsilyl enol ethers 3a–c were synthesized, and the kinetics of their reactions with the benzhydrylium ions 4 was studied by UV–vis spectroscopy in dichloromethane. Comparison with nonfluorinated analogues shows that replacement of CH3 by CF3 reduces the nucleophilic reactivity by 8 ... READ MORE
28-Nov-2012
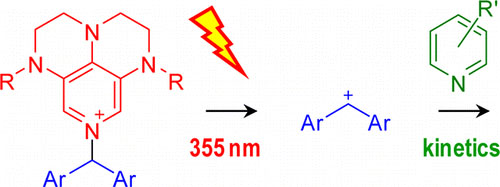
Laser flash irradiation of substituted N-benzhydryl pyridinium salts yields benzhydryl cations (diarylcarbenium ions) and/or benzhydryl radicals (diarylmethyl radicals). The use of 3,4,5-triamino-substituted pyridines as photoleaving groups allowed us to employ the third harmonic of a Nd/YAG laser (355 nm) for the photogeneration of benzhydryl cations. In ... READ MORE
28-Nov-2012
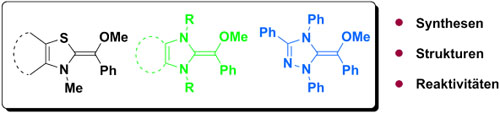
So nah wie möglich: Da Breslow-Intermediate üblicherweise in der Keto-Form vorliegen, können ihre O-geschützten Tautomeren als ihre engsten isolierbaren Verwandten angesehen werden. Ihre Synthese und Struktur sowie die Kinetik ihrer Reaktionen mit Elektrophilen wurden untersucht.
... READ MORE28-Nov-2012
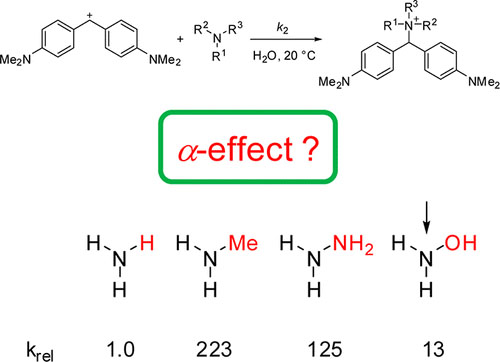
The kinetics of the reactions of amines, hydrazines, hydrazides, and hydroxylamines with benzhydrylium ions and quinone methides were studied in acetonitrile and water by UV–vis spectroscopy, using conventional spectrometers and stopped-flow and laser-flash techniques. From the second-order rate constants k2 of these reactions, the nucleophilicity ... READ MORE
28-Nov-2012
Second-order rate constants k2 for the reactions of various donor- and acceptor-substituted benzhydrylium ions Ar2CH+ with π-nucleophiles in CH2Cl2 were determined by laser flash irradiation of benzhydryl triarylphosphonium salts Ar2CH-PAr3+X– in the ... READ MORE
11-Oct-2012
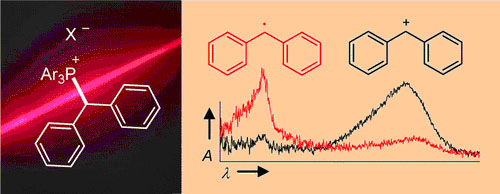
UV irradiation (266 or 280 nm) of benzhydryl triarylphosphonium salts ... UV irradiation (266 or 280 nm) of benzhydryl triarylphosphonium salts Ar2CH–PAr3+ X– yields benzhydryl cations Ar2CH+ and/or benzhydryl radicals Ar2CH·. Efficiency and mechanism of the photo-cleavage were studied by nanosecond laser flash photolysis and by ultrafast spectroscopy with a ... READ MORE
20-Aug-2012
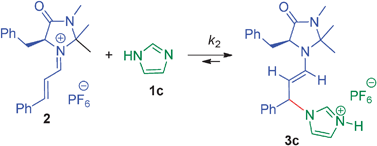
The imidazoles 1a–g add to the CC-double bond of the iminium ion 2 with rate constants as predicted by the equation log k = sN(N + E). Unfavourable proton shifts from the imidazolium unit to the enamine fragment in the adduct 3 account for the failure of imidazoles to take part in iminium-activated ... READ MORE
20-Aug-2012
The kinetics of the reactions of eleven substituted enamides with benzhydrylium ions (diarylcarbenium ions) were determined in acetonitrile solution. The second-order rate constants follow the correlation log k2(20°C)=sN(E+N), which allowed us to derive reactivity parameters N and ... READ MORE
01-Aug-2012
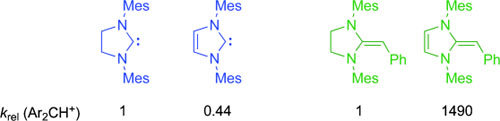
Aromatic or nonaromatic? Kinetic investigations show that structurally analogous saturated and unsaturated N-heterocyclic carbenes have almost identical nucleophilic reactivities, while the corresponding deoxy Breslow intermediates differ dramatically. ... READ MORE
01-Aug-2012
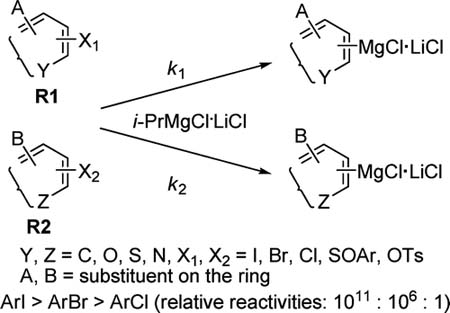
Relative reactivities and absolute rate constants of the reactions of haloarenes with i-PrMgCl·LiCl were investigated in THF at 0 °C. The rate of the halogen–magnesium exchange decreases in the series ArI > ArBr > ArCl (relative reactivities: 1011:106:1). Preliminary experiments show that the p-tolylsulfinyl group is ... READ MORE
01-Aug-2012
The nucleophile-specific parameters N and sN, as defined by log k20°C=sN(N+E) have been derived for the guanidines 1 a–h from the second-order rate constants of their reactions with diarylcarbenium tetrafluoroborates in ... READ MORE
01-Aug-2012
Kinetics of the reactions of bissulfonyl ethylenes with various carbanions, a sulfur ylide, and siloxyalkenes have been investigated photometrically at 20°C. The second-order rate constants have been combined with the known nucleophile- specific parameters N and sN for the nucleophiles to calculate the empirical electrophilicity ... READ MORE
01-Aug-2012
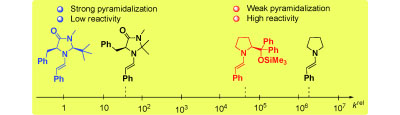
Extraordinarily weak nucleophiles: Enamines derived from imidazolidinones are 103–105 times less reactive than those derived from the Hayashi–Jørgensen catalyst. This finding explains the lower activity of MacMillan catalysts for enamine-activated reactions. ... READ MORE
01-Aug-2012
1,4 but not 1,2! The reactivity of 1 towards different nucleophiles (deprotonated β-diketones, enamines, and malonodinitrile) was investigated by NMR and kinetic experiments. These investigations proved that CC bond formation occurs by a Michael-type 1,4-addition and not by a 1,2-addition and subsequent [3,3]-sigmatropic ... READ MORE
01-Aug-2012
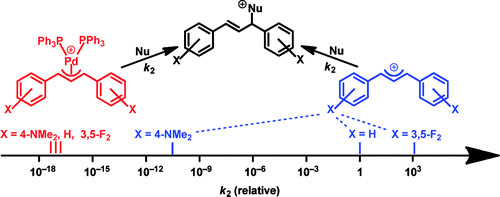
Kinetics of the reactions of [(η3-1,3-diarylallyl)Pd(PPh3)2]+ complexes with carbanions, enamines, amines, and triphenylphosphine have been investigated photometrically in dichloromethane, DMSO, and acetonitrile solutions at 20 °C. Amines were found to react both at palladium (substitution of PPh3) and at the ... READ MORE
11-Apr-2012
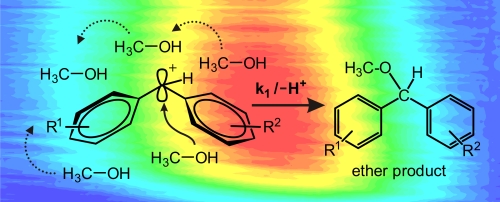
We combine broad-band femtosecond transient absorption measurements and on-the-fly molecular dynamics calculations to decipher the microscopic evolution of the geometry and solvation of photogenerated benzhydryl cations in bulk solution. The identification of the transition state or a short-lived intermediate of a chemical reaction is essential for the understanding ... READ MORE
27-Feb-2012
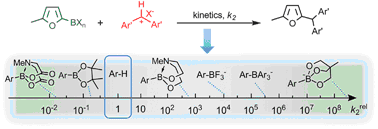
High nucleophilic reactivity and functional group tolerance are opposing properties of organometallic compounds. While organolithium compounds are highly nucleophilic and, therefore, react with most electrophiles, organosilicon compounds are on the low-reactivity end of the nucleophilicity scale. They tolerate numerous electrophilic functional groups and often ... READ MORE
27-Feb-2012
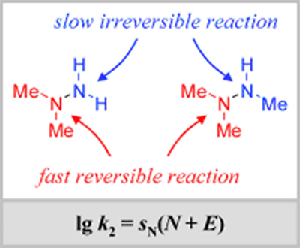
Kinetics versus thermodynamics: Methyl groups increase the nucleophilic reactivity of the substituted position of hydrazines and reduce the nucleophilicity of the adjacent nitrogen center. ... READ MORE
27-Feb-2012
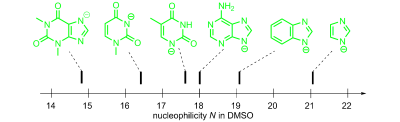
The kinetics of the reactions of different heterocyclic anions derived from imidazoles, purines, pyrimidines, and related compounds with benzhydrylium ions and structurally related quinone methides have been studied in DMSO and water ...
