Research Area B
Publications 2009
08-Nov-2010
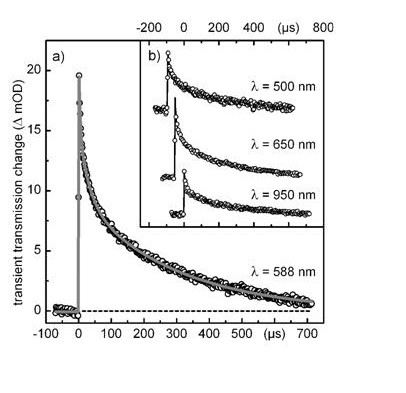
We show that the combination of a free-running 24 kHz supercontinuum fiber laser with a kHz femtosecond laser renders a sensitive and easy to implement transient spectrometer. It can directly extend the observation range of a standard broadband femtosecond spectrometer from the usual limit of a few nanoseconds to 1 millisecond and with the additional use of a ... READ MORE
08-Nov-2010
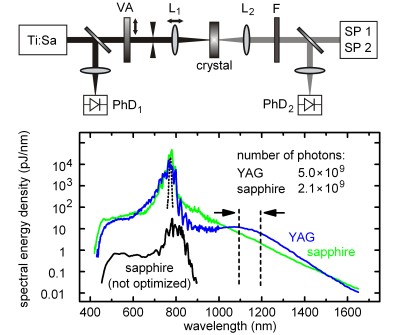
We report a comprehensive investigation of femtosecond continuum generation in single crystals of several common laser host materials. The absolute spectral energy density, pulse-to-pulse stability, pump threshold and beam profile are studied in dependence on the focusing conditions, crystal thickness, pump pulse energy and pump wavelength (775-1600 nm). Continuum ... READ MORE
08-Nov-2010
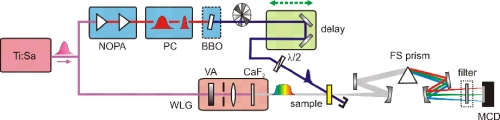
We give a full description of a state-of-the-art femtosecond transient spectrometer. The setup has been put together under full consideration of all technical and conceptual developments that became available in the last few years. Particular care was taken to avoid any unneeded components and modules. The spectrometer is operated at 1 kHz and based on a ... READ MORE
18-Jan-2010
Unsaturated benzyl cations have been generated laser-flash photolytically in acetonitrile in the presence of enol ethers or 2-methylfuran. The reactions of the cations with these π-nucleophiles follow second-order rate laws with rate constants comparable to those of the analogous saturated species. Product studies show the absence of cyclization products. In ... READ MORE
18-Jan-2010
Novel synthetic routes to the aryl-substituted quinone methides 3a–f have been developed, and a previously reported Mannich approach has been used for the syntheses of the acceptor substituted quinone methides 2e–g. The secondorder rate constants for the reactions of 3c–f and 2e–g with the carbanions 9a–h were determined photometrically in DMSO. With ... READ MORE
17-Nov-2009
Ordered mesoporous silica materials have been proposed as promising drug delivery systems and bone tissue regeneration precursors. In the present work, the behavior of colloidal mesoporous silica (CMS) nanoparticles was investigated in simulated biological fluid with the aim of gaining new insights about the reactivity of the CMS when brought into contact with ... READ MORE
23-Oct-2009
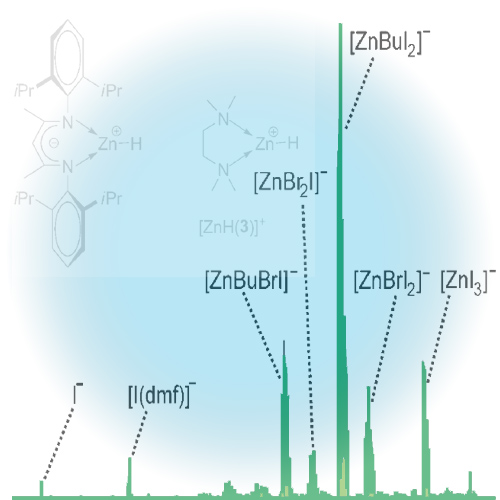
Solutions of butylzinc iodide in tetrahydrofuran, acetonitrile, and N,N-dimethylformamide were analyzed by electrospray ionization mass spectrometry. In all cases, microsolvated butylzinc cations [ZnBu(solvent)n]+, n=1-3, were detected. The parallel observation of the butylzincate anion [ZnBuI2]- suggests that these ions result from disproportionation of neutral ... READ MORE
03-Aug-2009
Bromine-magnesium exchange reactions of heteroaryl bromides provide an efficient entry to a wide variety of fiveand six-membered functionalized heteroaryl Grignard reagents, which can be used for the selective synthesis of polyfunctionalized heterocyclic compounds, such as thiazole, thiophene, furan, pyrrole, and pyridine derivatives.1,2 The resulting heteroarenes ... READ MORE
19-Jun-2009
The presence of LiCl considerably facilitates the insertion of magnesium into various aromatic and heterocyclic bromides. Several functional groups, such as -OBoc, -OTs, -Cl, -F,-CF3, -OMe, -NMe2, and -N2NR2, are well tolerated. The presence of a cyano group leads in some cases to competitive reduction of the organic halide to the corresponding ArH compound. The ... READ MORE
27-Apr-2009
Competition experiments have been performed to determine the relative reactivities of substituted bromobenzenes, bromonaphthalenes, and 9-bromoanthracene toward i-PrMgCl · LiCl in THF at 0 °C. The rates of the bromine-magnesium exchange reactions are accelerated by electron-acceptor substituents, the activating efficiency of which increases in the order para < ... READ MORE
27-Apr-2009
The rates of the reactions of the colored para-substituted phenylacetonitrile anions 1a-c and the phenylpropionitrile anions 2a-c with Michael acceptors (3a-u) were determined by UV-vis spectroscopy in DMSO at 20 °C. The reactions follow second-order kinetics, and the corresponding rate constants k2 obey the linear-free-energy relationship log k2(20 °C) ) s(N + E), ... READ MORE
16-Jan-2009
The kinetics of the reactions of azulene (1), 6,6-dimethylfulvene (2), 6-[4-(dimethylamino)phenyl]fulvene (3) and 6-(julolidin-9-yl)fulvene (4) with a set of benzhydrylium ions (reference electrophiles) have been investigated in MeCN. The second-order rate constants for these reactions correlate linearly with the electrophilicity parameters (E) of the benzhydrylium ... READ MORE
06-Jan-2009
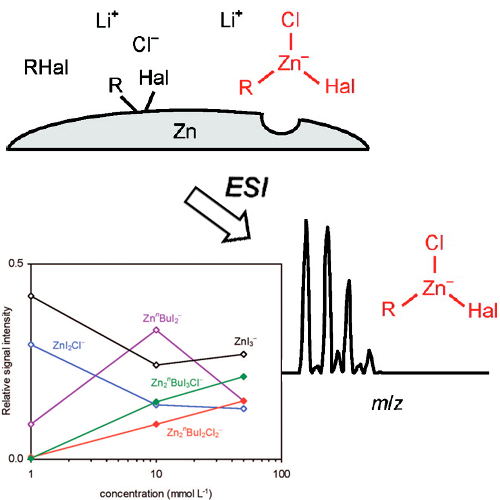
Tetrahydrofuran solutions of the products formed in LiCl-mediated zinc insertion reactions into various organic halides RHal were analyzed by anion-mode electrospray ionization (ESI) mass spectrometry. In all cases, organozincate anions were observed. The reactions with RHal, Hal ) Br and I, yielded predominantly mononuclear complexes, such as ZnRHal2 - and ... READ MORE
29-Dec-2008
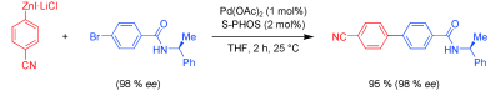
Mild mannered: Unprotected amides and sulfonamides containing aryl bromides and iodides undergo, in the presence of S-PHOS, smooth Negishi cross-couplings with various zinc organometallic reagents, allowing the preparation of pharmaceutically relevant molecules (see scheme; S-PHOS=2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl).
... READ MORE23-Dec-2008
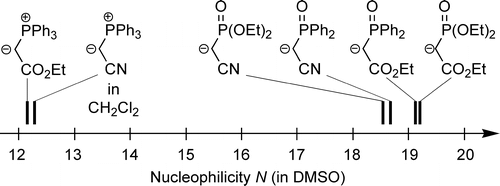
The kinetics of the reactions of four phosphoryl-stabilized carbanions 1a−d and four phosphorus ylides 1e−h with benzhydrylium ions 2a−h and structurally related quinone methides 2i−m have been determined by UV−vis spectroscopy. The second-order rate constants (k) correlated linearly with the electrophilicity parameters E of 2a−m, as required by the ... READ MORE
19-Dec-2008
A wide range of polyfunctional aryl, heteroaryl, alkyl, and benzylic zinc reagents were coupled with unsaturated aryl halides bearing an acidic NH or OH proton, using palladium(II) acetate (1 mol%), and S-Phos (2 mol%) as catalyst without the need of protecting groups. ... READ MORE
17-Dec-2008
A full functionalization of all four positions of the thiophene ring was achieved. Starting from readily available 2,5-dichlorothiophene, successive magnesiations of the 3- and 4-positions using TMPMgCl·LiCl furnish, after trapping with various electrophiles, 3,4-difunctionalized dichlorothiophenes. Subsequent dechlorination and metalation or magnesium insertion ... READ MORE
03-Dec-2008
The Kumada cross-coupling allows direct Pd-catalyzed carbon–carbon bond formation between unsaturated halides and organomagnesium reagents (without further transmetalation steps)[1, 2] and is therefore a highly atom-economical cross-coupling reaction.[3] Most of these cross-couplings follow a standard mechanism (oxidative addition, ligand exchange, reductive ... READ MORE


