Characterization of the nucleophilic reactivities of thiocarboxylate, dithiocarbonate and dithiocarbamate anions
30-Aug-2011
Org. Biomol. Chem. 2011, 9, 8046-8050., 2011, 9, 8046-8050 published on 30.08.2011
Org.Biomol.Chem.
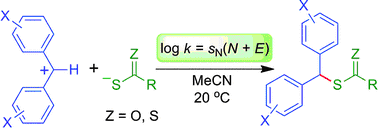
The kinetics of the reactions of thiocarboxylate and thiocarbonate anions with benzhydrylium ions have been determined in acetonitrile solution using laser-flash photolytic techniques. The second-order rate constants (k) correlate linearly with the electrophilicity parameters E of the benzhydrylium ions, as required by the correlation log k (20 Grad Celsius) = sN(N + E) (J. Am. Chem. Soc., 2001, 123, 9500–9512), allowing us to calculate the nucleophile-specific parameters N and sN for these anions. With these parameters, a direct comparison of the reactivities of thiocarboxylate, dithiocarbonate and dithiocarbamate anions with other nucleophiles becomes possible.