Effect of the “Supersilyl” Group on the Reactivities of Allylsilanes and Silyl Enol Ethers
26-Oct-2010
Org. Lett., 2010, 12, 5206–5209 published on 26.10.2010
Org. Lett.
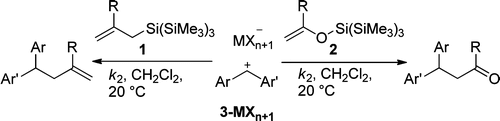
Kinetics of the reactions of allylsilanes (1) and silyl enol ethers (2) with benzhydrylium ions (3) were studied by UV−vis spectroscopy in dichloromethane at 20 °C. The less than three times higher reaction rates of the tris(trimethylsilyl)silyl compounds in comparison to the corresponding trimethylsilyl compounds indicate that the previously reported strong electron-donating effect of the supersilyl group operates only in the α-position and not in the β-position.