Oxidation State, Aggregation, and Heterolytic Dissociation of Allyl Indium Reagents
08-Apr-2010
JACS, 2010, 132 (17), 6032–6040 published on 08.04.2010
JACS, online article
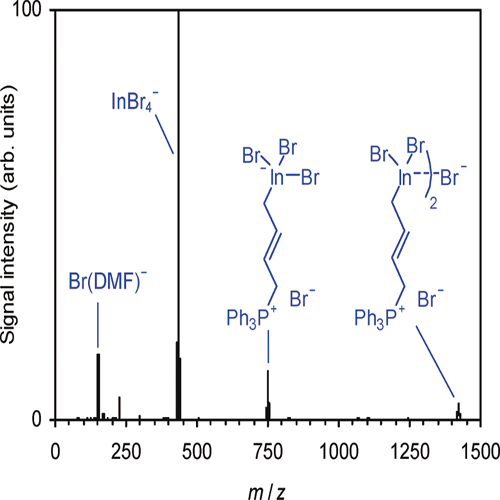
Solutions of allyl indium reagents formed in the reactions of indium with allyl bromide and allyl iodide, respectively, in N,N-dimethylformamide, tetrahydrofuran, and water were analyzed by a combination of electrospray-ionization mass spectrometry, temperature-dependent 1H NMR spectroscopy, and electrical conductivity measurements. Additional mass spectrometric experiments probed charge-tagged derivatives of the allyl indium reagents. The results obtained indicate the presence of allyl indium(+3) species, which undergo heterolytic dissociation to yield ions such as InR2(solv)+ and InRX3 - with R ) allyl and X ) Brand I. The extent of dissociation is greatest for N,N-dimethylformamide, whereas aggregation effects are more pronounced for the less polar tetrahydrofuran. The heterolytic dissociation of the allyl indium reagents supposedly enhances their reactivity by simultaneously providing highly Lewis acidic allyl indium cations and nucleophilic allyl indate anions.