Scope and Limitations of Cyclopropanations with Sulfur Ylides
29-Nov-2010
J. Am. Chem. Soc., 2010, 132, 17894–17900 published on 29.11.2010
J. Am. Chem. Soc.
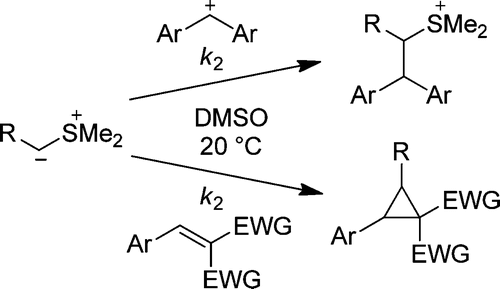
The rates of the reactions of the stabilized and semistabilized sulfur ylides 1a−g with benzhydrylium ions (2a−e) and Michael acceptors (2f−v) have been determined by UV−vis spectroscopy in DMSO at 20 °C. The second-order rate constants (log k2) of these reactions correlate linearly with the electrophilicity parameters E of the electrophiles 2 as required by the correlation log k2 = s(N + E), which allowed us to calculate the nucleophile-specific parameters N and s for the sulfur ylides 1a−g. The rate constants for the cyclopropanation reactions of sulfur ylides with Michael acceptors lie on the same correlation line as the rate constants for the reactions of sulfur ylides with carbocations. This observation is in line with a stepwise mechanism for the cyclopropanation reactions in which the first step, nucleophilic attack of the sulfur ylides at the Michael acceptors, is rate determining. As the few known pKaH values for sulfur ylides correlate poorly with their nucleophilic reactivities, the data reported in this work provide the first quantitative approach to sulfur ylide reactivity.