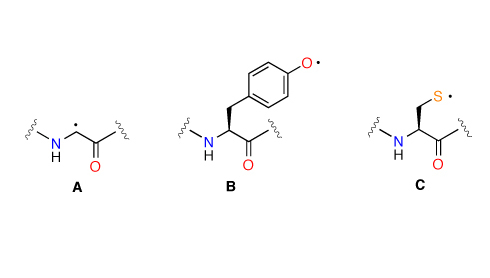
The Stability of Cα Peptide Radicals: Why Glycyl Radical Enzymes?
21-Jan-2011
Chem. Eur. J., 2011, published on 21.02.2011
Chem. Eur. J.
The conformational space of dipeptide models derived from glycine, alanine, phenylalanine, proline, tyrosine, and cysteine has been searched extensively and compared with the corresponding Cα dipeptide radicals at the G3(MP2)-RAD level of theory. The results indicate that the (least-substituted) glycine dipeptide radical is the thermochemically most stable of these species. Analysis of the structural parameters indicates that this is due to repulsive interactions between the Cα substituents and peptide units in the radical. A comparison of the conformational preferences of dipeptide radicals and their closed-shell parents also indicates that radical stability is a strongly conformation-dependent property.