Copper(I)-Mediated Oxidative Cross-Coupling between Functionalized Alkynyl Lithium and Aryl Magnesium Reagents
17-Oct-2007
Angew. Chem. Int. Ed., 2007, 46, 9093-96 published on 17.10.2007
Angew. Chem. Int. Ed., online article
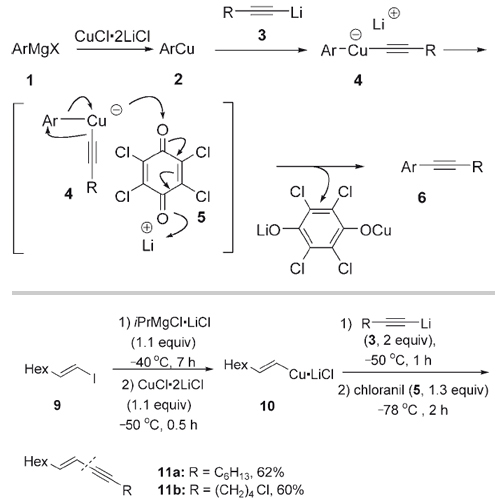
Polyfunctional alkynes are important intermediates for the preparation of natural products, pharmaceuticals, and organic materials such as molecular wires. The Sonogashira reaction and the Negishi cross-coupling are very powerful methods for preparing polyfunctional alkynes through the formation of a C(sp2)-C(sp) bond. However, the Sonogashira coupling fails for the selective monocoupling of dihaloarenes and dihaloalkenes as well as for the coupling of sterically shielded haloarenes. Recently, we reported an oxidative homocoupling of unsaturated magnesium or zinc reagents using a diphenoquinone or chloranil as oxidation agents. Soon after, we described a related oxidative amination procedure providing general synthesis of polyfunctional amines. Herein, we report a new type of oxidative coupling for the preparation of polyfunctional alkynes. Aryl or heteroaryl magnesium halides (ArMgX) are transmetalated to the corresponding copper reagents (ArCu) using the THF-soluble salt CuCl·2 LiCl. Subsequent reaction with an alkynyl lithium compound results in the formation of a mixed lithium aryl(alkynyl) cuprate. Its treatment with chloranil provides polyfunctional alkynes by an oxidative cross-coupling reaction.