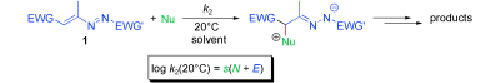Electrophilic Reactivities of 1,2-Diaza-1,3-dienes
10-Sep-2010
Chem. Eur. J., 2010, 16, 12008–12016 published on 18.10.2010
Chem. Eur. J.
The kinetics of the reactions of 1,2-diaza-1,3-dienes 1 with acceptor-substituted carbanions 2 have been studied at 20 °C. The reactions follow a second-order rate law, and can be described by the linear free energy relationship log k(20 °C)=s(N+E) [Eq. (1)]. With Equation (1) and the known nucleophile-specific parameters N and s for the carbanions, the electrophilicity parameters E of the 1,2-diaza-1,3-dienes 1 were determined. With E parameters in the range of −13.3 to −15.4, the electrophilic reactivities of 1 a–d are comparable to those of benzylidenemalononitriles, 2-benzylideneindan-1,3-diones, and benzylidenebarbituric acids. The experimental second-order rate constants for the reactions of 1 a–d with amines 3 and triarylphosphines 4 agreed with those calculated from E, N, and s, indicating the applicability of the linear free energy relationship [Eq. (1)] for predicting potential nucleophilic reaction partners of 1,2-diaza-1,3-dienes 1. Enamines 5 react up to 102 to 103 times faster with compounds 1 than predicted by Equation (1), indicating a change of mechanism, which becomes obvious in the reactions of 1 with enol ethers.