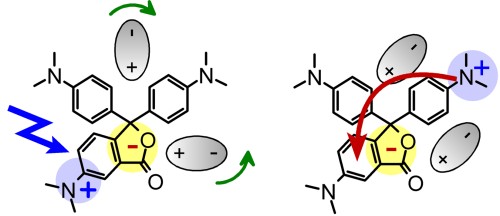
The Key Role of Solvation Dynamics in Intramolecular Electron Transfer: Time-Resolved Photophysics of Crystal Violet Lactone
22-Jun-2009
J. Phys. Chem. A, 2008, 112, 8487-8496 published on 21.08.2008
J. Phys. Chem. A
The intramolecular electron-transfer reaction in crystal violet lactone in polar aprotic solvents is studied with femtosecond transient absorption spectroscopy. The initially excited charge transfer state 1CTA is rapidly converted into a highly polar charge transfer state 1CTB. This ultrafast electron transfer is seen as a solventdependent dual fluorescence in steady-state spectra. We find that the electron-transfer process can be followed by a change from a double-peaked transient absorption spectrum to a single-peak one in the low picosecond range. The transient absorption kinetic curves are multiexponential, and the fitted time constants are solvent dependent but do not reproduce the known solvation times. For 6-dimethylaminophthalide, the optically active constituent of crystal violet lactone, only a small temporal evolution of the spectra is found. To explain these findings, we present a model that invokes a time-dependent electron-transfer rate. The rate is determined by the instantaneous separation of the two charge-transfer states. Because of their differing dipole moments, they are dynamically lowered to a different extent by the solvation. When they temporarily become isoenergetic, equal forward and backward transfer rates are reached. The intrinsic electron-transfer (1CTA → 1CTB) reaction is probably as fast as that in the structurally analogous malachite green lactone (on the 100 fs time scale). The key element for the dynamics is therefore its control by the solvent, which changes the relative energetics of the two states during the solvation process. With further stabilization of the more polar state, the final equilibrium in state population is reached.