Buildup and Decay of the Optical Absorption in the Ultrafast Photo-Generation and Reaction of Benzhydryl Cations in Solution
11-Apr-2012
J.Phys.Chem.A, 2012, 116, 11064-11074 published on 11.04.2012
J.Phys.Chem.A
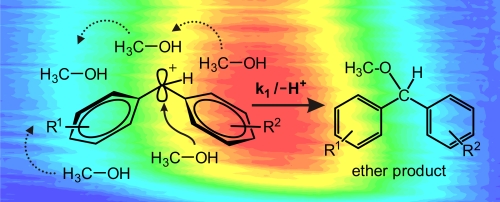
We combine broad-band femtosecond transient absorption measurements and on-the-fly molecular dynamics calculations to decipher the microscopic evolution of the geometry and solvation of photogenerated benzhydryl cations in bulk solution. The identification of the transition state or a short-lived intermediate of a chemical reaction is essential for the understanding of the mechanism. For a direct identification typically transient optical spectroscopy is used, preferentially with high temporal resolution. We combine broad-band femtosecond transient absorption measurements and on-the-fly molecular dynamics calculations to decipher the microscopic evolution of the geometry and solvation of photogenerated benzhydryl cations (Ar2CH+, Ar = phenyl, p-tolyl, m-fluorophenyl, or m,m′-difluorophenyl) in bulk solution. From the high level quantum chemical calculations on the microsolvated cation we can deduce a narrowing and blue shift of the cation absorption that is nearly quantitatively equal to the experimental finding. The roughly 300 fs initial increase in the absorption signal found for all investigated combinations of benzhydryl chlorides or phosphonium salts as benzhydryl cation precursors and solvents is therefore assigned to the planarization and solvation of the nascent fragment of the bond cleavage. The actual cleavage time cannot directly be deduced from the rise of the spectroscopic signal. For alcohols as solvent, the cation combines on the picosecond time scale either with one of the solvent molecules to the ether or to a lesser degree geminately with the leaving group. The study shows that the absorption signal attributable to a species like the benzhydryl cation does not mirror the concentration during the first instances of the process. Rather, the signal is determined by the geometrical relaxation of the photoproduct and the response of the solvent.