Radicals in enzymatic catalysis— a thermodynamic perspective
22-Sep-2009
Faraday Discuss., 2010, 145, 301 - 313 published on 22.09.2009
Faraday Discuss., online article
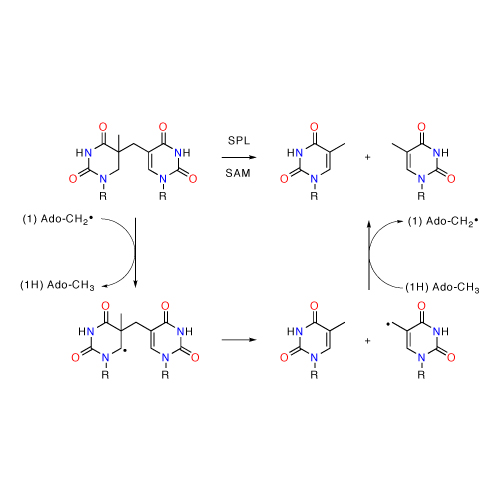
The thermodynamic stability of radicals involved in enzymatic catalysis has been quantified using a series of theoretical methods. It is found that three of the most often encountered radicals located on the enzyme protein chain (tyrosyl, cysteinyl and glycyl radicals) are of similar stability. This is despite the fact that O–H, S–H and C–H bonds have intrinsically very different homolytic bond dissociation energies. The cofactor-derived C5´-adenosyl radical, in contrast, is significantly less stable than these protein-bound radicals.